A substantial body of research describes the anticancer potential of dichloroacetate (DCA), however, its successful therapeutic administration in cancer treatment is currently restricted to clinical trials. The presence of adverse effects such as neurotoxicity as well as the possibility of DCA carcinogenicity still hinders the clinical usage of DCA.
However, in the past years, the number of publications supporting DCA employment against cancer expanded also because of the enormous interest in targeting the metabolism of tumour cells. Dissecting the DCA mode of action helps to grasp the roots of its selective efficiency against cancer cells.
Effective coadministration of DCA cancer testimonials with conventional chemotherapy, radiation, other medicines, or natural substances has been explored in numerous cancer models. New drug delivery technologies and multi-action compounds comprising DCA and other medications tend to improve bioavailability and look more efficient owing to the synergistic action of several agents.
The proliferation of findings indicating the effectiveness of DCA in cancer treatment has encouraged other investigations that allowed to uncover other possible molecular targets of DCA. Interestingly, DCA might greatly influence cancer stem cell fraction and lead to cancer elimination. Collectively, these data give a solid argument for new clinical translational research on DCA in cancer treatment.
A possible mechanism of action for DCA in cancer
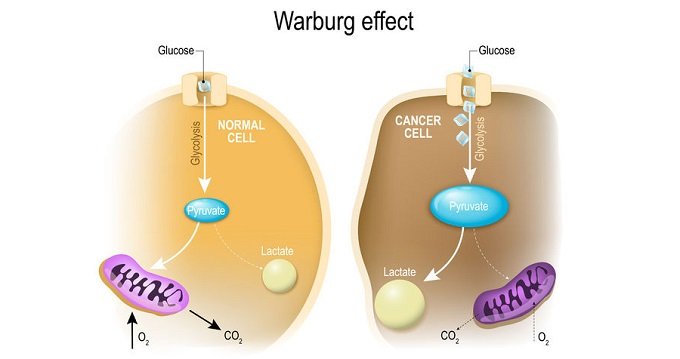
In cancer treatment, DCA may be effective because of the metabolic features of tumours, such as increased glycolytic activity and decreased mitochondrial oxidation independent of oxygen supply, the Warburg effect. In the tumour microenvironment, acidosis is caused by an overabundance of lactate produced as a consequence of uncontrolled glycolysis. Lactate, a byproduct of glycolysis, is taken up by surrounding cells to aid tumour development and prevent apoptosis.
In cancer cells, the increase of enzymes involved in glycolysis leads to the inhibition of apoptosis. DCA is a potent inhibitor of the enzyme pyruvate dehydrogenase-kinase (PDK), which is essential for cancer cells to switch from glycolysis to the oxidation of pyruvate. As a result of PDH activation, cancer cells lose their edge in the metabolic arena.
The development and the spreading
Malignant cells are unable to meet their energy needs because of mitochondrial DNA abnormalities, which often develop during carcinogenesis and result in respiratory chain failure. Lactate generation is also reduced by DCA cancer testimonials, which affects the acidosis condition in the tumour microenvironment, limiting tumour development and spreading. The enhanced outflow of cytochrome c and other apoptosis-inducing substances and the elevation of ROS levels that follow from the introduction of pyruvate into mitochondria reduce the survival of cancer cells.
When it comes to the low amounts of disinfection by-products present in drinking water, DCA is a possible carcinogen. Early-life exposure to DCA in mice has been linked to an increased risk of hepatocellular tumours, according to research. DCA’s long-term impacts on cellular metabolism might have epigenetic consequences. Long-term PDH and other oxidative processes associated with glucose metabolism might increase reactive oxygen species and mitochondrial stress if they are inactivated.
